Molar Mass of O
Therefore the molar mass of a substance calculated by adding the atomic masses of each element present in that molecule. Molecular mass molecular weight is the mass of one molecule of a substance and is expressed in the unified atomic mass units u.

Boc Sciences Provides Molecular Weight Calculator Use For Molar Mass Molar Mass Teaching Chemistry Chemistry Lessons
The molar mass of water for example is roughly 18015 gmol which is the mass of NA number of water molecules.
. 4 Be Beryllium 90121831. The molar mass of oxygen is 1600 gmol. Next multiply the number of a particular element by its.
Find the atomic mass for each element by using the mass given in the Periodic TableMultiply the subscript number of atoms times the atomic mass of that element and add the masses of all of the elements in the molecule to get the molecular mass. C 6 H 10 O. Let us calculate the molar mass of NaCl compound.
3 x 16 48. Chemical formula Hill notation Molar mass gmol Modify Clear mnM. The Molar volume is directly proportional to molar mass and inversely proportional to density.
The SI unit of molar mass is kgmol. To find the molecular mass add the atomic masses of all of the atoms in the molecule. Molar Mass of Glucose C 6 H 12 O 6 180156.
Only three countries - Burma Liberia and the United States - have yet to adopt the International System of Units as their official system of measurement weights and measures. 3 Li Lithium 694. CuCl 2 CuCl2 C 12 H 22 O 11 C12H22O11 C 6 H 5 3 PCCO C18H15PCCO Formula.
47 C 53 F. Therefore the molecular mass of the given compound is. Calculate the molecular mass of Ammonium Sulphate NH 4 2 SO 4.
226 K Boiling point. Atomic mass of Oxygen 1600. The percentage by weight of any atom or group of atoms in a compound can be computed by dividing the total weight of the atom or group of atoms in the formula by the formula weight and multiplying by 100.
The molar mass of zinc nitrate will be equal to 1 atom x 65 gramsmole of zinc two atoms x 14 gramsmole of nitrogen six atoms x 16 gramsmole of oxygen 189 gramsmole of zinc nitrate. The calculated value is numerically identical to 1 u or 1. 1 u is equal to 112 the mass of one atom of carbon-12.
Well add those numbers together along with the unit grams per mole for finding molar mass. Convert Dates Salary Chemistry. If the formula used in calculating molar mass is the molecular formula the formula weight computed is the molecular weight.
7 N Nitrogen 14007. The formula of the molar volume is expressed as beginarraylV_m fracMolar massDensityendarray Where V m is the volume of the substance. 2 He Helium 4002602.
Uses the formula of a reactant to determine molar mass. This map shows international measuring system of units and the chronology of the advance of metric usage around the world. The molar mass of hydrogen is 1008 gmol as given by the period table.
Calculation of Molar Mass. You can also use our. Atomic mass of Carbon 1201.
From this you can see that sodiums molar mass will be 2299 gmol. The above formula can also be expressed in terms of the mass fraction w i. 10 Ne Neon 201797.
Therefore the molar mass of Na2CO3 is 106 gmole. We can also use molecular weight calculator for finding molar mass of a. Enter formulas with proper capitalization and unpack brackets.
Start by determining how many of each elements there are by looking the subscripts small number next to the element symbol. The molar mass equation. Basically you should know how to find the molar masses of any chemical compound now.
Mass grams g But your mass isnt given in grams. 5 B Boron 1081. The molar mass of an element is the atomic mass of the element.
9 F Fluorine 18998403163. 2 x 140 28. The molar mass of oxygen is 1600 gmol.
The unit of molar mass in the SI system is kilogram per mole. This way we can calculate the molar mass of a compound or one-carbon compound. 8 O Oxygen 15999.
The mass of the sample containing about 6 023 1 0 23 6023 times 10 23 6 023 1 0 23 atoms or molecules see Avogadro number. If we round off the. Examples of molecular weight computations.
1 x 401 401. Since calcium nitrate contains one atom of calcium two atoms of nitrogen and six atoms of oxygen. A common request on this site is to.
For all other compounds the general idea is the same. Molar mass Mass Moles. The percentage by weight of any atom or group of atoms in a compound can be computed by dividing the total weight of the atom or group of atoms in the formula by the formula weight and multiplying by 100.
Peppermint or acetone-like. When we add up the total values ie 46 12 48 106. 09478 gmL liquid Melting point.
Identify the molar mass of calcium nitrate CaNO 3 2. Otherwise the molecular formula is a multiple of the empirical formula eg CH 2 O is. In general we write the unit as gmol.
Since the relative atomic masses of N 14 H 1 S 32 O 16. Its as simple as that. Molar mass of Carbon Monoxide 2801.
Dont worry why dont you take some time to discover how to properly convert between different densities and weights. The standard temperature used is 273 Kelvin or 0 o C Standard pressure is 1 atmosphere ie 760 mm Hg. 6 C Carbon 12011.
M mix is the molar mass of a mixture of n components and x i and M i are the mole fraction and the molar mass of i th component. Molar Mass of Mixtures. The molar mass is simply the mass of one mole of substance ie.
9815 gmol Appearance Colorless liquid Odor. If the formula used in calculating molar mass is the molecular formula the formula weight computed is the molecular weight. For example the molar mass of calcium-40 is 39962 590 98 22 gmol whereas the molar mass of calcium-42 is 41958 618 01 27 gmol and of calcium with the normal isotopic mix is 400784 gmol.
Your result will show in gmol. The molar mass of a substance depends not only on its molecular formula but also on the distribution of isotopes of each chemical element present in it. In this compound there are 1 C 4 H 31 and 1 O.
The molar mass of a mixture is determined using the mole fraction x i. Definitions of molecular mass molecular weight molar mass and molar weight. Select elements and see the molar mass of the compound.
In some cases the empirical formula is the same as the molecular formula which gives the actual number of atoms in a compound eg H 2 O.
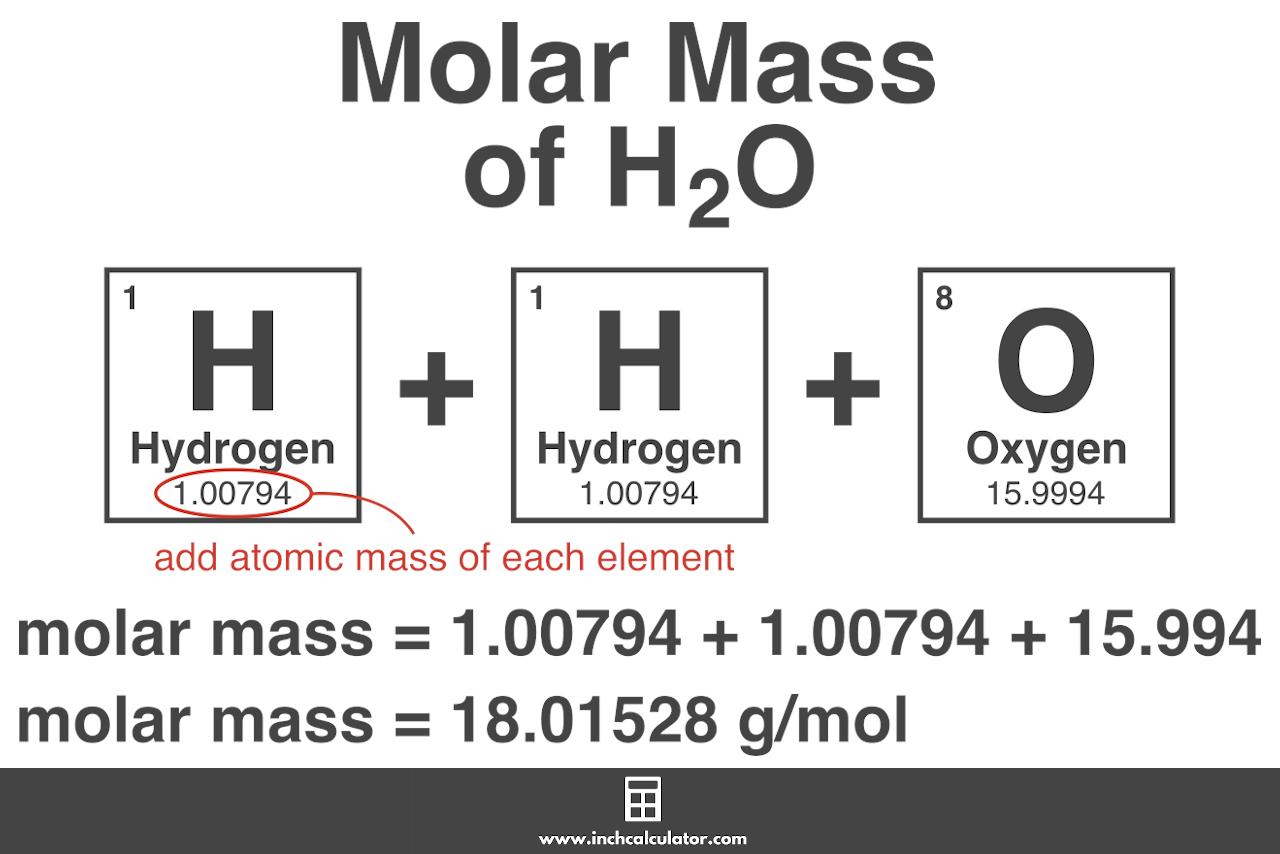
Molar Mass Calculator Inch Calculator
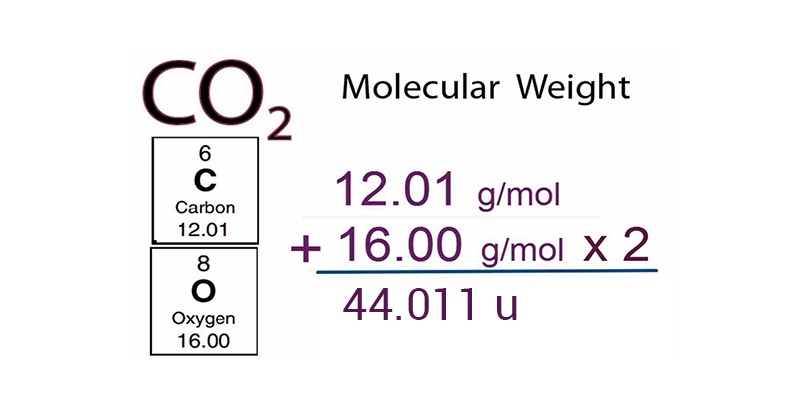
Atomic And Molecular Mass Elements Relative Molecular Weight

Molar Mass Definition Formula In 2022 Molar Mass Chemistry Basics Relative Atomic Mass
Comments
Post a Comment